
These stabilized species are more commonly found in the environment at low temperatures. Ions are also produced in the liquid or solid state when salts interact with solvents (for example, water) to produce solvated ions, which are more stable, for reasons involving a combination of energy and entropy changes as the ions move away from each other to interact with the liquid. Ions in their gas-like state are highly reactive and will rapidly interact with ions of opposite charge to give neutral molecules or ionic salts. Arrhenius' explanation was that in forming a solution, the salt dissociates into Faraday's ions, he proposed that ions formed even in the absence of an electric current. Svante Arrhenius put forth, in his 1884 dissertation, the explanation of the fact that solid crystalline salts dissociate into paired charged particles when dissolved, for which he would win the 1903 Nobel Prize in Chemistry. In correspondence with Faraday, Whewell also coined the words anode and cathode, as well as anion and cation as ions that are attracted to the respective electrodes.

This conveys matter from one place to the other. Faraday did not know the nature of these species, but he knew that since metals dissolved into and entered a solution at one electrode and new metal came forth from a solution at the other electrode that some kind of substance has moved through the solution in a current.

This term was introduced (after a suggestion by the English polymath William Whewell) by English physicist and chemist Michael Faraday in 1834 for the then-unknown species that goes from one electrode to the other through an aqueous medium. They are so called because ions move toward the electrode of opposite charge. A cation is something that moves down ( Greek: κάτω pronounced kato, meaning "down") and an anion is something that moves up ( Greek: ano ἄνω, meaning "up"). The word ion was coined from Greek neuter present participle of ienai ( Greek: ἰέναι), meaning "to go". Ions are also created by chemical interactions, such as the dissolution of a salt in liquids, or by other means, such as passing a direct current through a conducting solution, dissolving an anode via ionization.
NEGATIVELY CHARGED ION FREE
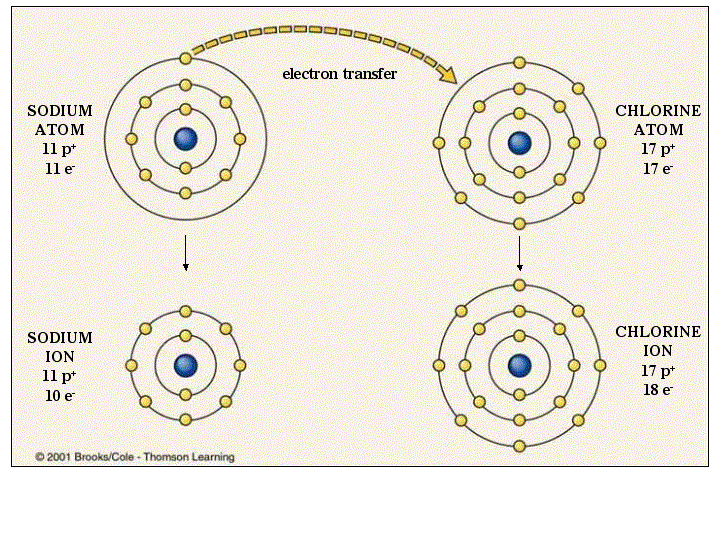
In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a positive ion. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.Ī cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. ɒ n, - ən/) is an atom or molecule with a net electrical charge. Forming an ionic bond, Lithium and Fluorine become Li+ and F- ions.Īn ion ( / ˈ aɪ. Electron transfer between Lithium and Fluorine.


 0 kommentar(er)
0 kommentar(er)
